QUELL - Trademark Details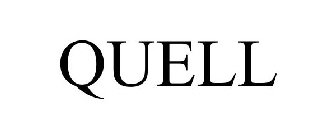
Status: 700 - Registered
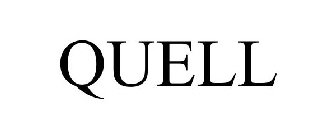
Serial Number
88372290
Registration Number
6077717
Word Mark
QUELL
Status
700 - Registered
Status Date
2020-06-16
Filing Date
2019-04-04
Registration Number
6077717
Registration Date
2020-06-16
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2020-03-31
Attorney Name
Law Office Assigned Location Code
M40
Employee Name
BAYLISS, HUNTER A
Statements
Goods and Services
Pharmaceutical and biological preparations for diagnostic, medical, therapeutic, and prophylactic use for inducing tolerance to a transplant and for treating, preventing or ameliorating transplant rejection, Graft-versus-Host Disease (GvHD), autoimmune and allergic disease, undesired inflammation and inflammatory disease, and for promoting tissue repair and regeneration; veterinary preparations for diagnostic, medical, therapeutic, and prophylactic use for inducing tolerance to a transplant and for treating, preventing or ameliorating transplant rejection, Graft-versus-Host Disease (GvHD), autoimmune and allergic disease, undesired inflammation and inflammatory disease, and for promoting tissue repair and regeneration; preparations for cell therapy, namely, preparations comprising T cells having an immunosuppressive phenotype; preparations for gene therapy; pharmaceutical, medical, and clinical preparations for use in treatment, amelioration and prophylaxis of immune system related diseases and disorders; preparations for therapies to modulate the immune system for inducing tolerance to a transplant and for treating, preventing or ameliorating transplant rejection, Graft-versus-Host Disease (GvHD), autoimmune and allergic disease, undesired inflammation and inflammatory disease, and for promoting tissue repair and regeneration; preparations for therapies for autoimmune conditions and immunological disorders; cancer preparations, namely, chimeric antigen receptors and therapeutic preparations thereof for inducing tolerance to a transplant and for treating, preventing or ameliorating transplant rejection, Graft-versus-Host Disease (GvHD), autoimmune and allergic disease, undesired inflammation and inflammatory disease, and for promoting tissue repair and regeneration; diagnostic kits consisting of monoclonal antibodies for use in disease testing and for inducing tolerance to a transplant and for treating, preventing or ameliorating transplant rejection, Graft-versus-Host Disease (GvHD), autoimmune and allergic disease, undesired inflammation and inflammatory disease, and for promoting tissue repair and regeneration; preparations for therapeutic delivery of nucleic acids for inducing tolerance to a transplant and for treating, preventing or ameliorating transplant rejection, Graft-versus-Host Disease (GvHD), autoimmune and allergic disease, undesired inflammation and inflammatory disease, and for promoting tissue repair and regeneration; viral or nucleic acid vectors for therapeutic or prophylactic purposes and preparations containing such vectors for inducing tolerance to a transplant and for treating, preventing or ameliorating transplant rejection, Graft-versus-Host Disease (GvHD), autoimmune and allergic disease, undesired inflammation and inflammatory disease, and for promoting tissue repair and regeneration; preparations for genetically modifying human and animal cells for diagnostic and medical purposes; preparations of blood or cells extracted from humans or animals which have been adapted for therapeutic purposes; none of the foregoing having any relation to pain treatment or management
Goods and Services
Manufacturing and processing pharmaceuticals for others; manufacturing and processing biological, biotechnological and biopharmaceutical preparations for others; processing of biological tissue, blood, cell, and genetic material for others; production and manufacture of viral vectors for others; production and manufacture of gene therapy products for others; custom manufacture of pharmaceutical and biological preparations for others; none of the foregoing having any relation to pain treatment or management
Goods and Services
advisory services relating to gene therapy and design; Scientific and technological services, namely, research and design in the field of T cells with an immunosuppressive phenotype and transplant rejection, Graft-versus-Host Disease (GvHD), autoimmune and allergic disease, undesired inflammation and inflammatory disease, and for promoting tissue repair and regeneration; industrial analysis and research in the field of cell therapy; medical and veterinary research services in the field of the manipulation of cells within a laboratory or research facility to enable the cells to be used for therapeutic purposes; scientific research in the development of new therapies in the field of autoimmune, immunological disorders and to modulate the immune system; scientific research in human and animal cell therapy; scientific research in human and animal gene therapy; scientific research into vectors and preparations for manipulating and adapting human and animal cells; scientific and technological services, namely, design in the field of cell therapy; none of the foregoing having any relation to pain treatment or management
Goods and Services
Medical services; medical services in the field of immunology; medical services in the field of surgery; medical services in the field of autoimmune diseases and conditions; medical services, in the field of, tissue transplant surgery; collection and preservation of biological tissue, blood, cells and genetic materials; medical diagnosis of transplant rejection, Graft-versus-Host Disease (GvHD), autoimmune and allergic disease, undesired inflammation and inflammatory disease, and for promoting tissue repair and regeneration; Medical treatment of transplant rejection, Graft-versus-Host Disease (GvHD), autoimmune and allergic disease, undesired inflammation and inflammatory disease, and for promoting tissue repair and regeneration; providing information and data for diagnosis and medical treatment of transplant rejection, Graft-versus-Host Disease (GvHD), autoimmune and allergic disease, undesired inflammation and inflammatory disease, and for promoting tissue repair and regeneration; gene and nucleic acid delivery or modification for therapeutic purposes; medical treatment, namely, administering cell or gene therapies; advisory services in the field of health as it relates to cell or gene therapy; medical advisory services relating to cell or gene therapy; none of the foregoing having any relation to pain treatment or management
Classification Information
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
6 - Active
Class Status Date
2019-04-22
Primary Code
005
International Class
040 - Treatment of materials. - Treatment of materials.
US Class Codes
100, 103, 106
Class Status Code
6 - Active
Class Status Date
2019-04-22
Primary Code
040
International Class
042 - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services. - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services.
US Class Codes
100, 101
Class Status Code
6 - Active
Class Status Date
2019-04-22
Primary Code
042
International Class
044 - Medical services; veterinary services; hygienic and beauty care for human beings or animals; agriculture, horticulture and forestry services. - Medical services; veterinary services; hygienic and beauty care for human beings or animals; agriculture, horticulture and forestry services.
US Class Codes
100, 101
Class Status Code
6 - Active
Class Status Date
2019-04-22
Primary Code
044
Current Trademark Owners
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
99 - Other (limited company (ltd.)).
Address
Please log in with your Justia account to see this address.
Trademark Owner History
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
99 - Other (limited company (ltd.)).
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
99 - Other (limited company (ltd.)).
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
10 - Original Applicant
Legal Entity Type
99 - Other (limited company (ltd.)).
Address
Please log in with your Justia account to see this address.
Correspondences
Name
Steven T. Shelton
Address
Please log in with your Justia account to see this address.
Foreign Application Information
| Filing Date | Application Number | Country | Foreign Priority Claim In |
| 2019-04-04 | 018048095 | EU | True |
Trademark Events
| Event Date | Event Description |
| 2019-04-08 | NEW APPLICATION ENTERED IN TRAM |
| 2019-04-22 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2019-06-14 | ASSIGNED TO EXAMINER |
| 2019-06-24 | NON-FINAL ACTION WRITTEN |
| 2019-06-24 | NON-FINAL ACTION E-MAILED |
| 2019-06-24 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2019-12-09 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2019-12-30 | ASSIGNED TO LIE |
| 2019-12-30 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2019-12-30 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2020-01-23 | NON-FINAL ACTION WRITTEN |
| 2020-01-23 | NON-FINAL ACTION E-MAILED |
| 2020-01-23 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2020-02-13 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2020-02-13 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2020-02-13 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2020-02-25 | EXAMINERS AMENDMENT -WRITTEN |
| 2020-02-25 | EXAMINERS AMENDMENT E-MAILED |
| 2020-02-25 | NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED |
| 2020-02-25 | EXAMINER'S AMENDMENT ENTERED |
| 2020-02-25 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2020-03-11 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2020-03-31 | PUBLISHED FOR OPPOSITION |
| 2020-03-31 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2020-06-16 | REGISTERED-PRINCIPAL REGISTER |