ADRIEN GAGNON - Trademark Details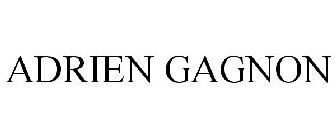
Status: 734 - Fifth Extension - Granted
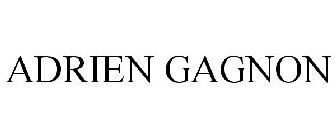
Serial Number
88025012
Word Mark
ADRIEN GAGNON
Status
734 - Fifth Extension - Granted
Status Date
2021-10-01
Filing Date
2018-07-03
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2019-01-29
Attorney Name
Law Office Assigned Location Code
L40
Employee Name
PARK, JENNY K
Statements
Goods and Services
Goods: glucosamine sulphate and chondroitin formula, chitosan oligosaccharides, MSM in the form of tablets for relieving joint pain and symptoms related to arthritis and osteoarthritis and for contributing to the formation of healthy cartilage; plant extracts in the form of capsules for improving the health, for reducing the frequency of climateric episodes (hot flashes), improving the neurovegetative state associated with menopause, improving blood cholesterol levels, alleviating excessive perspiration and enabling regulation of a disrupted sleep cycle; Natural products for human use, namely, meal replacements in the form of meal replacement bars which taste like peanuts, crème caramel, fruit, chocolate, apple-cinnamon, coffee, granola and chocolate chips to assist in weight loss and weight maintenance; Natural products for human use, namely, a vitamin and mineral supplement formula to remedy vitamin and mineral deficiencies; plant extract formulas with calming antioxidant properties, plant extract formulas possessing immune system benefits, to help balance mood swings, aiming at palliating the reduction of estrogen in menopausal women, antioxidants in the form of tablets, for maintaining good health; Natural products for human use, namely, plant extracts in the form of capsules, with purifying, eliminative, diuretic and laxative actions; Natural products for human use, namely, oligosaccharide chitosan capsules assisting in controlling cholesterol; Natural products for human use, namely, chitosan oligosaccharide ampoules and creams to soothe joint pain and act as anti-inflammatories for joints; Natural products for human use, namely, vitamin supplement formulas in the form of gelcaps for maintaining an appropriate level of antioxidants in the body to palliate oxidative stress; vitamin supplement formulas in the form of tablets to assist in balancing human metabolism, namely, carbohydrate levels and to assist in reducing homocysteine levels; mineral formulas in the form of capsules and tablets for remedying magnesium deficiencies; Natural products for human use, namely, a supplement formula made of vitamins, plants and adaptogenic roots in the form of energizing ampoules, to decrease hyperglycemia and stimulate weakened immune systems and cognitive functions; Natural products for human use, namely, plant and root extracts in the form of capsules and ampoules to assist in preventing and treating urinary tract infections, soothing respiratory symptoms stemming from a cold or flu, treating moderate Alzheimer's, facilitating digestion of food and to assist in reducing symptoms associated with digestive disorders; plant, root, and bark extracts in capsule form for treating lumbar pain, arthritis pain, rheumatic disorders; plant extracts in capsule form for soothing respiratory symptoms due to a cold or flu, the formula of which has proven to be an immuno-stimulant in preclinical studies; leaf, root, and bark extracts in the form of infusions, and capsules with a purifying, eliminative, diuretic and laxative action; Natural products for human use, namely, leaf, flower, and bark extracts in the form of capsules for combatting sleep disorders and reducing nervousness; Natural products for human use, namely, a vitamin supplement and antioxidant fruit extract formula in the form of capsules to assist in decreasing the duration and severity of cold symptoms, slowing down the development of atherosclerosis, improving blood circulation and blood pressure; a food supplement formula in the form of gelcaps containing soya lecithin and carthane oil to prevent memory disorders and hypercholesterolemia; plant extracts in the form of capsules to assist in fighting hypertension or hypercholesterolemia; a vitamin and mineral food supplement formula in the form of tablets to stimulate the appetite, treat acne and furunculosis; oils in the form of gelcaps rich in fatty acids and vitamin E for prostate health; Natural products for human use, namely, plant extracts and antioxidant roots and fish oils in the form of capsules, gelcaps, liquids, to assist in reducing the damage caused by oxidative stress, decreasing the risk of prostate cancer and lung cancer, coronary illnesses, to assist in treating rheumatoid arthritis, to improve muscular strength and endurance and to accelerate recovery after an injury, to soothe urological symptoms associated with benign prostate hyperplasia, to combat general fatigue, to assist in preventing macular degeneration associated with age, cataracts, the maintenance of proper retina functioning, the maintenance of cardiovascular and cognitive health, for preventing and treating urinary tract infections, to assist in diminishing the risks of prostate cancer and lung cancer, to assist in reducing the symptoms of light to moderate depression, improving memory and immune system activity; Natural products for human use, namely, yoghurt culture in the form of capsules contributing to the regulation of intestinal flora, stimulating the immune system; Natural products for human use, namely, potassium gluconate in the form of tablets for preventing hypokalemia and hypertension; Natural products for human use, namely, an essentially fatty acid formula in the form of gelcaps to assist in fighting eczema, premenstrual syndrome and menopausal symptoms; Natural products for human use, namely, chitin formula made from crustacean shells, organic beef trachea, chicken sternum extracts and standardized resin extracts in the form of capsules and tablets to assist in soothing osteoarthritis symptoms, to reduce joint pain, protect against cartilage deterioration resulting from chronic joint disorders and play a role in the maintenance and building of cartilage, diminish the pain associated with arthritis, assist in slowing the progression of joint erosion, reduce the risk of osteoporosis in arthritic patients; Natural products for human use, namely, a vitamin and mineral supplement formula in the form of capsules, tablets and ampoules for soothing the symptoms of menopause, relaxing muscles, reducing nervous tension, reducing premenstrual syndrome symptoms, decreasing edema, reducing the volume/circumference of the legs, decreasing pain and tension in the legs, maintaining bone mass, preventing osteoporosis, fighting fatigue associated with anemia, preventing anemia; Natural products for human use, namely, gel in the form of ampoules to combat aging; Natural products for human use, namely, soluble fibres in the form of capsules for intestinal regularity; Natural products for human use, namely, plant and mineral extracts in the form of capsules and liquids to promote intestinal regularity and to aid in digestion and purify (detoxify); Natural products for human use, namely, mineral formulas in the form of capsules and tablets to remedy calcium deficiencies
Classification Information
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
6 - Active
Class Status Date
2018-07-09
Primary Code
005
Current Trademark Owners
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
16 - Limited Liability Company
Address
Please log in with your Justia account to see this address.
Trademark Owner History
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
16 - Limited Liability Company
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
10 - Original Applicant
Legal Entity Type
16 - Limited Liability Company
Address
Please log in with your Justia account to see this address.
Correspondences
Name
Joseph V. Myers III
Address
Please log in with your Justia account to see this address.
Prior Registrations
| Relationship Type | Reel Number |
| Continuity Child | 88976227 |
Trademark Events
| Event Date | Event Description |
| 2018-07-09 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2018-07-23 | TEAS VOLUNTARY AMENDMENT RECEIVED |
| 2018-07-23 | TEAS AMENDMENT ENTERED BEFORE ATTORNEY ASSIGNED |
| 2018-10-23 | ASSIGNED TO EXAMINER |
| 2018-10-30 | NON-FINAL ACTION WRITTEN |
| 2018-10-30 | NON-FINAL ACTION E-MAILED |
| 2018-10-30 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2018-12-10 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2018-12-14 | ASSIGNED TO LIE |
| 2018-12-19 | ASSIGNED TO LIE |
| 2018-12-19 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2018-12-19 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2018-12-19 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2019-01-09 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2019-01-29 | PUBLISHED FOR OPPOSITION |
| 2019-01-29 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2019-03-26 | NOA E-MAILED - SOU REQUIRED FROM APPLICANT |
| 2019-09-25 | TEAS EXTENSION RECEIVED |
| 2019-09-25 | EXTENSION 1 FILED |
| 2019-09-25 | EXTENSION 1 GRANTED |
| 2019-09-27 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2019-10-29 | TEAS REQUEST TO DIVIDE RECEIVED |
| 2019-11-12 | CASE ASSIGNED TO INTENT TO USE PARALEGAL |
| 2019-10-29 | DIVISIONAL REQUEST RECEIVED |
| 2019-11-12 | DIVISIONAL PROCESSING COMPLETE |
| 2019-11-13 | CORRECTED NOA E-MAILED |
| 2019-11-14 | TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED |
| 2019-11-14 | ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED |
| 2020-03-10 | TEAS EXTENSION RECEIVED |
| 2020-03-10 | EXTENSION 2 FILED |
| 2020-03-10 | EXTENSION 2 GRANTED |
| 2020-03-12 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2020-09-25 | TEAS EXTENSION RECEIVED |
| 2020-09-25 | EXTENSION 3 FILED |
| 2020-09-25 | EXTENSION 3 GRANTED |
| 2020-09-29 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2021-03-09 | TEAS EXTENSION RECEIVED |
| 2021-03-09 | EXTENSION 4 FILED |
| 2021-03-09 | EXTENSION 4 GRANTED |
| 2021-03-11 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2021-09-22 | TEAS EXTENSION RECEIVED |
| 2021-09-22 | EXTENSION 5 FILED |
| 2021-10-01 | EXTENSION 5 GRANTED |
| 2021-10-02 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |