GEOMX - Trademark Details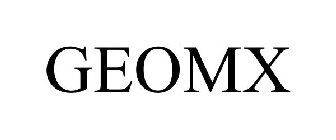
Status: 807 - SU - Non-Final Action - Mailed
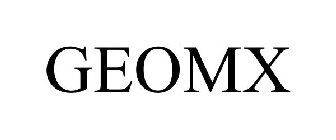
Serial Number
87914539
Word Mark
GEOMX
Status
807 - SU - Non-Final Action - Mailed
Status Date
2021-12-03
Filing Date
2018-05-09
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2018-12-11
Attorney Name
Law Office Assigned Location Code
O30
Employee Name
RATCLIFFE, ROBERT L
Statements
Goods and Services
reagents and instruments for scientific, research or non-medical or veterinary diagnostic use in the fields of pathology, immunohistochemistry, in situ-hybridization, proteomics, RNA transcription, RNA translation, gene expression profiling and nucleic acid sequencing; diagnostic reagents for scientific or research use in the fields of cancer, neurology and infectious disease; reagents for scientific, research or non-medical or veterinary diagnostic use relating to molecular identification and digital quantification; reagents for scientific, research or non-medical or veterinary diagnostic use containing antibodies and nucleic acids; reagent kits comprising primarily reagents for scientific, research or non-medical or veterinary diagnostic use in the fields of cancer, neurology and infectious disease; reagents for immuno-histochemistry detection and in situ-hybridization detection for scientific, research or non-medical or veterinary diagnostic use; test kits for scientific, research or non-medical or veterinary diagnostic use comprising reagents, slides, solid matrix materials, microfluidic devices and support documentation, namely, instructional manuals, therefor; test kits comprising reagents for scientific, research or non-medical or veterinary diagnostic use relating to molecular identification and digital quantification; reagents for in vitro use in clinical chemistry and molecular biology; reagents for clinical or medical laboratory use, namely, reagents for pathology, immunohistochemistry, in situ-hybridization, proteomics, RNA transcription, RNA translation, gene expression profiling, nucleic acid sequencing, and clinical, oncology, neurology, and infectious disease diagnostics; multiparameter quantitative detection and analysis products and technology for research and diagnostics for a variety of uses in the health care and other life science sectors, namely, reagents for scientific or medical research use, diagnostic preparations and reagents for scientific or research uses, namely, pathology, immunohistochemistry, in situ-hybridization, proteomics, RNA transcription, RNA translation, gene expression profiling and nucleic acid sequencing; test kits, consisting of chemical preparations for scientific purposes, in particular for the detection, diagnosis, progress monitoring and progress prediction of diseases, including inflammations, infections, disorders of the central nervous systems, cardiovascular, neurological, endocrine, autoimmune and genetic disorders and cancers
Goods and Services
diagnostic reagents and instruments for medical or veterinary use in the fields of pathology, immunohistochemistry, in situ-hybridization, proteomics, RNA transcription, RNA translation, gene expression profiling and nucleic acid sequencing; diagnostic reagents for medical or veterinary use in the fields of cancer, neurology and infectious disease; diagnostic reagent kits comprising primarily of diagnostic reagents for medical or veterinary use in the fields of cancer, neurology and infectious disease; chemical reagents for medical use; reagents for medical or veterinary use containing antibodies and nucleic acids; diagnostic test kits for medical use comprising primarily reagents, slides, solid matrix materials, microfluidic devices sold as a unit with instructional manuals therefor; medical or veterinary diagnostic kits comprising medical or veterinary diagnostic reagents for use in detecting the presence of analytes in samples and identifying sample type, and equipment, namely, medical diagnostic immuno-assays for screening samples detecting the presence of analytes in samples and identifying sample type, sold as a unit with instructional manuals; diagnostic preparations in the nature of antibodies, for medical and veterinary purposes; reagents and diagnostic reagents for medical or veterinary purposes; diagnostic preparations in the nature of in situ hybridization probes, for medical and veterinary purposes; medical or veterinary diagnostic reagents for pathology, immunohistochemistry, in situ-hybridization, proteomics, RNA transcription, RNA translation, gene expression profiling and nucleic acid sequencing; multiparameter quantitative detection and analysis products and technology for diagnostics for a variety of uses in health care, namely, pathology, immunohistochemistry, in situ-hybridization, proteomics, RNA transcription, RNA translation, gene expression profiling and nucleic acid sequencing; medical diagnostic reagents and assays for testing of body fluids or tissue; kits consisting primarily of medical or veterinary diagnostic reagents and assays for testing of body fluids or tissue; reagents in the form of cell markers and molecule markers including antibodies and nucleic acids for medical or veterinary purposes; medical diagnostic reagents for use in the fields of pathology, immunohistochemistry, in situ-hybridization, proteomics, RNA transcription, RNA translation, gene expression profiling and nucleic acid sequencing; medical diagnostic reagents for use relating to molecular identification and digital quantification; medical diagnostic reagents containing antibodies and nucleic acids; medical diagnostic reagents for use in the fields of cancer, neurology and infectious disease; medical diagnostic reagents for immuno-histochemistry detection and in situ-hybridization detection; medical diagnostic reagents for use relating to molecular identification and digital quantification
Goods and Services
automated apparatus in the nature of automated laboratory equipment and computer systems comprised of computer hardware and software for detection and analysis of biomolecules for scientific use; automated apparatus in the nature of automated laboratory equipment and computer systems comprised of computer hardware and software for scientific research, namely, for detecting, collecting and processing optical signals and images, into data for use in the analysis of biomolecules in connection with scientific or medical research; automated apparatus in the nature of automated laboratory equipment in the nature of digital imagers, digital displays, microscopes, robotics, fluidic stations and computer systems for staining and/or visualization of tissue or cell based samples for diagnostic or scientific use; automated apparatus in the nature of automated laboratory equipment in the nature of digital imagers, digital displays, microscopes, robotics and computer systems for digitizing images of biological tissue for use in connection with pathology; automated apparatus in the nature of automated laboratory equipment in the nature of digital imagers, digital displays, microscopes, robotics and computer systems for capturing digital images of microscope slides of biological tissue, storage of digitized microscope slide images in a data storage area, and display of digitized microscope slide images; automated apparatus in the nature of automated laboratory equipment in the nature of digital imagers, digital displays, microscopes, robotics, fluidic stations and computer systems comprised of computer hardware and software for detection and analysis of, pathology, gene expression, RNA transcription, RNA translation and nucleic acid sequencing; computer hardware and software to enable pathologists to diagnose disease; computer hardware and software for the electronic scanning of clinical pathology slides; computer hardware and software for pathologists to render and report diagnoses and results of scientific research; computer hardware and software for scientific or research uses, namely, pathology, immunohistochemistry, in situ-hybridization, proteomics, RNA transcription and microRNA expression profiling and nucleic acid sequencing; computer hardware and software for digitizing, processing and storing images of biological tissues and cells; computer hardware and software for analyzing the results of tissues and cell based samples obtained through operation of tissue and cell sample staining and visualization apparatus; computer hardware and software for identifying and diagnosing cancer and other diseases and processing digital images; computer hardware and software for viewing, analyzing, managing, reporting, and archiving digital images; laboratory equipment for sample preparation, mixing, hybridization, incubation, washing, location and imaging, namely, robotic equipment, fluid mixers, pumps, valves, and containers, reagent holders, sample containers and microfluidic devices, optical devices, scanning microscopes, fluorescent microscopes, digital micromirror devices, electromagnetic sensors and replacement parts therefor in the fields of scientific research; computer systems, namely, software and data files to acquire, analyze, manage and report biological information
Goods and Services
clinical and medical diagnostic instruments, namely, histopathology instruments and nucleic acid sequencers; clinical and medical diagnostic instruments for analysis of tissue and/or cell based samples, all for medical purposes; clinical and medical diagnostic instruments for performing immunohistochemistry and in situ-hybridization staining reactions, histological and pathological staining and image processing and analysis; histopathology instruments for clinical purposes; clinical and medical diagnostic instruments for immunohistochemistry and in situ-hybridization analyses; clinical and medical diagnostic instruments for the electronic scanning of clinical pathology slides, processing and storing images of biological tissues and cells and digitizing, processing and storing images of biological tissues and cells
Goods and Services
research and product development services for others in the field of biotechnology; medical and scientific research services for others in the fields of pathology, immunohistochemistry, in situ-hybridization, proteomics, RNA transcription, RNA translation, gene expression profiling and nucleic acid sequencing; medical and scientific research in the field of cancer treatment and diagnosis; medical and scientific research services for others in the field of infectious disease; medical and scientific research services for others in the field of neurology; medical and scientific research services for others, namely, research and design in the field of testing, analyzing and examining biological samples, medical samples, and cell samples; providing laboratory research services for others in the field of gene expression and genetic data mining; research and development services for others for acquiring, analyzing, and managing biological and genetic information in the fields of diagnostic reagents, forensics, medicine, genetics, and environment; research, design, and development services for others in the fields of diagnostic reagents, compounds and devices, measuring apparatus for use in product research and development, separation and purification processes
Classification Information
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
005, 006, 018, 044, 046, 051, 052
Class Status Code
6 - Active
Class Status Date
2018-05-18
Primary Code
005
First Use Anywhere Date
2018-09-05
First Use In Commerce Date
2018-09-05
Current Trademark Owners
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Trademark Owner History
Correspondences
Name
Alyssa Worsham
Address
Please log in with your Justia account to see this address.
Prior Registrations
| Relationship Type | Reel Number |
| Continuity Child | 87982200 |
Madrid International Filings
Entry Number
1
Reference Number
A0117112
Original Filing Date USPTO
2021-12-08
International Status Code
403
| Madrid History Events | ||
| Date | Code | Description |
| 2021-12-08 | NEWAP | NEW APPLICATION FOR IR RECEIVED |
| 2021-12-13 | MCERT | MANUALLY CERTIFIED |
Trademark Events
| Event Date | Event Description |
| 2018-05-12 | NEW APPLICATION ENTERED IN TRAM |
| 2018-05-18 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2018-08-30 | ASSIGNED TO EXAMINER |
| 2018-08-31 | PRIORITY ACTION WRITTEN |
| 2018-08-31 | PRIORITY ACTION E-MAILED |
| 2018-08-31 | NOTIFICATION OF PRIORITY ACTION E-MAILED |
| 2018-10-14 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2018-10-14 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2018-10-15 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2018-10-18 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2018-11-02 | ASSIGNED TO LIE |
| 2018-11-02 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2018-11-21 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2018-12-11 | PUBLISHED FOR OPPOSITION |
| 2018-12-11 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2019-02-05 | NOA E-MAILED - SOU REQUIRED FROM APPLICANT |
| 2019-08-15 | TEAS PETITION TO REVIVE RECEIVED |
| 2019-08-15 | PETITION TO REVIVE-GRANTED |
| 2019-08-15 | EXTENSION RECEIVED WITH TEAS PETITION |
| 2019-08-15 | NOTICE OF REVIVAL - E-MAILED |
| 2019-10-02 | CASE ASSIGNED TO INTENT TO USE PARALEGAL |
| 2019-08-05 | EXTENSION 1 FILED |
| 2019-10-02 | EXTENSION 1 GRANTED |
| 2019-10-03 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2019-10-23 | TEAS REQUEST TO DIVIDE RECEIVED |
| 2019-10-25 | CASE ASSIGNED TO INTENT TO USE PARALEGAL |
| 2019-10-23 | DIVISIONAL REQUEST RECEIVED |
| 2019-10-28 | DIVISIONAL PROCESSING COMPLETE |
| 2019-10-29 | CORRECTED NOA E-MAILED |
| 2020-01-30 | TEAS EXTENSION RECEIVED |
| 2020-01-30 | EXTENSION 2 FILED |
| 2020-01-30 | EXTENSION 2 GRANTED |
| 2020-02-01 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2020-07-20 | TEAS EXTENSION RECEIVED |
| 2020-07-20 | EXTENSION 3 FILED |
| 2020-07-20 | EXTENSION 3 GRANTED |
| 2020-07-22 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2021-01-20 | TEAS EXTENSION RECEIVED |
| 2021-01-20 | EXTENSION 4 FILED |
| 2021-01-20 | EXTENSION 4 GRANTED |
| 2021-01-22 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2021-08-05 | TEAS STATEMENT OF USE RECEIVED |
| 2021-08-05 | TEAS EXTENSION RECEIVED |
| 2021-08-05 | EXTENSION 5 FILED |
| 2021-08-16 | EXTENSION 5 GRANTED |
| 2021-08-05 | USE AMENDMENT FILED |
| 2021-08-16 | STATEMENT OF USE PROCESSING COMPLETE |
| 2021-08-17 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2021-08-19 | ASSIGNED TO EXAMINER |
| 2021-08-30 | ASSIGNED TO EXAMINER |
| 2021-12-03 | SU - NON-FINAL ACTION - WRITTEN |
| 2021-12-03 | NON-FINAL ACTION E-MAILED |
| 2021-12-03 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |