ENIVA HEALTH - Trademark Details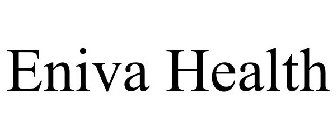
Status: 700 - Registered
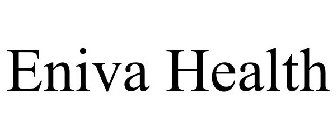
Serial Number
87656105
Registration Number
5778506
Word Mark
ENIVA HEALTH
Status
700 - Registered
Status Date
2019-06-18
Filing Date
2017-10-23
Registration Number
5778506
Registration Date
2019-06-18
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2019-04-02
Law Office Assigned Location Code
N60
Employee Name
BHUPATHI, TARA LUISE
Statements
Disclaimer with Predetermined Text
"HEALTH"
Goods and Services
Beverages containing chlorophyll for use as a nutritional supplement; Calcium supplements; Dietary supplement for eliminating toxins from the intestinal tract; Dietary supplemental drinks; Dietary supplemental drinks in the nature of vitamin and mineral beverages; Dietary supplements; Dietary supplements consisting primarily of Purified Water, Grape, Pomegranate, Aronia chokeberry, Cranberry, Carrot, Blueberry, Stevia leaf, Oregano, Elderberry, Acerola, Tomato, Lime, Lemon, Apple, Blackcurrant, Hibiscus flower, Pumpkin, Cherry, Grape Seed Extract, Wolfberry gojiberry, Orange, Citrus Bioflavonoids, Blackberry, Raspberry, Strawberry, Açai Berry, D-Ribose, Malic Acid, Green Tea Leaf Extract, L-Lysine, L-Proline, Glucosamine HCl vegetable, Alanine, Valine, Isoleucine, Glycine, Leucine, Fish Oil, Natural Extracts and flavors, Natural Sugars Beet, Molasses, Glycerin Vegetable, Magnesium Citric Acid, Potassium, Ascorbic Acid, Calcium, Natural Gums Xanthan, Guar, Arabic, D-Alpha Tocopherol Acetate with Mixed Tocopherols, Sorbic Benzoic acids, Niacin, Zinc, D-Calcium Pantothenate, L-Carnitine Fumarate, Retinyl Palmitate, Boron, Folic Acid, Glucosamine Hydrochloride vegetable, Manganese, Cholecalciferol, Pyridoxine Hydrochloride, Riboflavin Phosphate, Thiamine Hydrochloride, Isolated Soy Lecithin, Chromium, Biotin, Sorbimacrogol, Coenzyme Q10 ubiquinone, Selenium, Vanadium, Cyanocobalamin, N-Methyl Cobalamin, Mixed Tocopherols, Vitamin A, Vitamin C, Vitamin D, Vitamin E, Thiamin Vitamin B1, Riboflavin Vitamin B2, Niacin, Vitamin B6, Vitamin B12, Biotin, Pantothenic Acid, Phosphorus, Iodine, Copper, Melatonin, L-Theanine Valerian Extract valeriana officinalis, Chamomile Flower Extract chamomilla recutita, Passion Flower Extract passiflora incarnata, Lemon Balm Extract melissa officinalis, Aloe Vera Gel aloe barbadensis, Ashwagandha withania somnifera, EPA Eicosapentaenoic Acid, DHA Docosahexaenoic Acid, Lutein, Zeaxanthin, Fungal Pancreatin, Papain, Bromelain, Protease, Peptidase, Rutin, Saw Palmetto Fruit Extract, Beta-Sitosterol Phytosterols, Pygeum Africanum Bark Extract, Cranberry Extract, Stinging Nettle Root Extract, Pumpkinseed Oil, Evening Primrose Seed Oil, Lactobacillus Acidophilus, Lactobacillus Salivarius, Lactobacillus Plantarum, Bifidobacterium Infantis, Bifidobacterium Bifidum, Streptococcus Thermophilus, FOS Fructooligosaccharides, Inulin Jerusalem Artichoke Tuber, Chondroitin, Pineapple Extract, MSM, White Willow Extract, Collagen Type II, Mango Extract, Trans Resveratrol, Melatonin; Dietary supplements for animals; Dietary supplements for controlling cholesterol; Dietary supplements for human consumption; Dietary supplements for humans and animals; Dietary supplements for pets; Dietary supplements for Skin problems, poor energy levels, obesity, insomnia, heart issues, muscle issues, poor vision, macula degeneration, indigestion, prostate problems, bone issues, liver and intestinal problems; Dietary supplements in the nature of weight loss powders; Dietary and nutritional supplements; Dietary and nutritional supplements containing Purified Water, Grape, Pomegranate, Aronia chokeberry, Cranberry, Carrot, Blueberry, Stevia leaf, Oregano, Elderberry, Acerola, Tomato, Lime, Lemon, Apple, Blackcurrant, Hibiscus flower, Pumpkin, Cherry, Grape Seed Extract, Wolfberry gojiberry, Orange, Citrus Bioflavonoids, Blackberry, Raspberry, Strawberry, Açai Berry, D-Ribose, Malic Acid, Green Tea Leaf Extract, L-Lysine, L-Proline, Glucosamine HCl vegetable, Alanine, Valine, Isoleucine, Glycine, Leucine, Fish Oil, Natural Extracts and flavors, Natural Sugars Beet, Molasses, Glycerin Vegetable, Magnesium Citric Acid, Potassium, Ascorbic Acid, Calcium, Natural Gums Xanthan, Guar, Arabic, D-Alpha Tocopherol Acetate with Mixed Tocopherols, Sorbic Benzoic acids, Niacin, Zinc, D-Calcium Pantothenate, L-Carnitine Fumarate, Retinyl Palmitate, Boron, Folic Acid, Glucosamine Hydrochloride vegetable, Manganese, Cholecalciferol, Pyridoxine Hydrochloride, Riboflavin Phosphate, Thiamine Hydrochloride, Isolated Soy Lecithin, Chromium, Biotin, Sorbimacrogol, Coenzyme Q10 ubiquinone, Selenium, Vanadium, Cyanocobalamin, N-Methyl Cobalamin, Mixed Tocopherols, Vitamin A, Vitamin C, Vitamin D, Vitamin E, Thiamin Vitamin B1, Riboflavin Vitamin B2, Niacin, Vitamin B6, Vitamin B12, Biotin, Pantothenic Acid, Phosphorus, Iodine, Copper, Melatonin, L-Theanine Valerian Extract valeriana officinalis, Chamomile Flower Extract chamomilla recutita, Passion Flower Extract passiflora incarnata, Lemon Balm Extract melissa officinalis, Aloe Vera Gel aloe barbadensis, Ashwagandha withania somnifera, EPA Eicosapentaenoic Acid, DHA Docosahexaenoic Acid, Lutein, Zeaxanthin, Fungal Pancreatin, Papain, Bromelain, Protease, Peptidase, Rutin, Saw Palmetto Fruit Extract, Beta-Sitosterol Phytosterols, Pygeum Africanum Bark Extract, Cranberry Extract, Stinging Nettle Root Extract, Pumpkinseed Oil, Evening Primrose Seed Oil, Lactobacillus Acidophilus, Lactobacillus Salivarius, Lactobacillus Plantarum, Bifidobacterium Infantis, Bifidobacterium Bifidum, Streptococcus Thermophilus, FOS Fructooligosaccharides, Inulin Jerusalem Artichoke Tuber, Chondroitin, Pineapple Extract, MSM, White Willow Extract, Collagen Type II, Mango Extract, Trans Resveratrol, Melatonin; Dietary and nutritional supplements for endurance sports; Dietary fiber for use as an ingredient in the manufacture of dietary supplements; Dietary food supplements; Enzyme dietary supplements; Enzyme food supplements; Food supplements; Food supplements, namely, anti-oxidants; Health food supplements; Herbal supplements; Herbal supplements for sleeping problems; Lecithin dietary supplements; Lecithin for use as a dietary supplement; Liquid herbal supplements; Liquid nutritional supplement; Liquid vitamin supplements; Mineral supplements; Mineral food supplements; Mineral nutritional supplements; Natural supplements for treating candida; Natural supplements for treating erectile dysfunction; Natural dietary supplements; Natural dietary supplements for the treatment of Skin problems, poor energy levels, obesity, insomnia, heart issues, muscle issues, poor vision, macula degeneration, indigestion, prostate problems, bone issues, liver and intestinal problems; Natural herbal supplements; Nutraceuticals for use as a dietary supplement; Nutraceuticals for use as a dietary supplement for Skin problems, poor energy levels, obesity, insomnia, heart issues, muscle issues, poor vision, macula degeneration, indigestion, prostate problems, bone issues, liver and intestinal problems; Nutritional supplement for eliminating toxins from the intestinal tract; Nutritional supplement shakes; Nutritional supplements; Nutritional supplements consisting primarily of Purified Water, Grape, Pomegranate, Aronia chokeberry, Cranberry, Carrot, Blueberry, Stevia leaf, Oregano, Elderberry, Acerola, Tomato, Lime, Lemon, Apple, Blackcurrant, Hibiscus flower, Pumpkin, Cherry, Grape Seed Extract, Wolfberry gojiberry, Orange, Citrus Bioflavonoids, Blackberry, Raspberry, Strawberry, Açai Berry, D-Ribose, Malic Acid, Green Tea Leaf Extract, L-Lysine, L-Proline, Glucosamine HCl vegetable, Alanine, Valine, Isoleucine, Glycine, Leucine, Fish Oil, Natural Extracts and flavors, Natural Sugars Beet, Molasses, Glycerin Vegetable, Magnesium Citric Acid, Potassium, Ascorbic Acid, Calcium, Natural Gums Xanthan, Guar, Arabic, D-Alpha Tocopherol Acetate with Mixed Tocopherols, Sorbic Benzoic acids, Niacin, Zinc, D-Calcium Pantothenate, L-Carnitine Fumarate, Retinyl Palmitate, Boron, Folic Acid, Glucosamine Hydrochloride vegetable, Manganese, Cholecalciferol, Pyridoxine Hydrochloride, Riboflavin Phosphate, Thiamine Hydrochloride, Isolated Soy Lecithin, Chromium, Biotin, Sorbimacrogol, Coenzyme Q10 ubiquinone, Selenium, Vanadium, Cyanocobalamin, N-Methyl Cobalamin, Mixed Tocopherols, Vitamin A, Vitamin C, Vitamin D, Vitamin E, Thiamin Vitamin B1, Riboflavin Vitamin B2, Niacin, Vitamin B6, Vitamin B12, Biotin, Pantothenic Acid, Phosphorus, Iodine, Copper, Melatonin, L-Theanine Valerian Extract valeriana officinalis, Chamomile Flower Extract chamomilla recutita, Passion Flower Extract passiflora incarnata, Lemon Balm Extract melissa officinalis, Aloe Vera Gel aloe barbadensis, Ashwagandha withania somnifera, EPA Eicosapentaenoic Acid, DHA Docosahexaenoic Acid, Lutein, Zeaxanthin, Fungal Pancreatin, Papain, Bromelain, Protease, Peptidase, Rutin, Saw Palmetto Fruit Extract, Beta-Sitosterol Phytosterols, Pygeum Africanum Bark Extract, Cranberry Extract, Stinging Nettle Root Extract, Pumpkinseed Oil, Evening Primrose Seed Oil, Lactobacillus Acidophilus, Lactobacillus Salivarius, Lactobacillus Plantarum, Bifidobacterium Infantis, Bifidobacterium Bifidum, Streptococcus Thermophilus, FOS Fructooligosaccharides, Inulin Jerusalem Artichoke Tuber, Chondroitin, Pineapple Extract, MSM, White Willow Extract, Collagen Type II, Mango Extract, Trans Resveratrol, Melatonin; Nutritional supplements for Humans, animals, supporting healthy skin, energy levels, weight loss, better sleep, heart health, muscle health, vision, indigestion, bone health, liver and intestinal cleansing; Nutritional supplements in capsule form for dogs; Nutritional supplements in the form of Liquid, pills, capsules, tablets, gels and powders; Nutritional supplements, namely, probiotic compositions; Prebiotic supplements; Probiotic supplements; Protein supplement shakes; Protein supplements; Protein dietary supplements; Vitamin supplements; Vitamin and mineral supplements; Vitamins and dietary food supplements for animals; Weight management supplements; Whey protein supplements
Classification Information
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
6 - Active
Class Status Date
2017-11-01
Primary Code
005
First Use Anywhere Date
2015-07-01
First Use In Commerce Date
2015-09-01
Current Trademark Owners
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Trademark Owner History
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
10 - Original Applicant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Correspondences
Name
ENIVA HEALTH
Address
Please log in with your Justia account to see this address.
Prior Registrations
| Relationship Type | Reel Number |
| Prior Registration | 4480053 |
| Prior Registration | 4840548 |
| Prior Registration | 4840885 |
Trademark Events
| Event Date | Event Description |
| 2017-10-26 | NEW APPLICATION ENTERED IN TRAM |
| 2017-11-01 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2018-02-01 | ASSIGNED TO EXAMINER |
| 2018-02-07 | NON-FINAL ACTION WRITTEN |
| 2018-02-07 | NON-FINAL ACTION E-MAILED |
| 2018-02-07 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2018-09-11 | ABANDONMENT - FAILURE TO RESPOND OR LATE RESPONSE |
| 2018-09-11 | ABANDONMENT NOTICE MAILED - FAILURE TO RESPOND |
| 2018-09-13 | TEAS PETITION TO REVIVE RECEIVED |
| 2018-09-13 | PETITION TO REVIVE-GRANTED |
| 2018-09-14 | NOTICE OF REVIVAL - E-MAILED |
| 2018-09-19 | NON-FINAL ACTION WRITTEN |
| 2018-09-19 | NON-FINAL ACTION E-MAILED |
| 2018-09-19 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2019-02-19 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2019-02-19 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2019-02-25 | ASSIGNED TO LIE |
| 2019-02-26 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2019-02-26 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2019-02-27 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2019-03-13 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2019-04-02 | PUBLISHED FOR OPPOSITION |
| 2019-04-02 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2019-06-18 | REGISTERED-PRINCIPAL REGISTER |