Image Trademark with Serial Number 86753205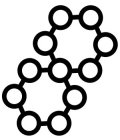
Status: 700 - Registered

Serial Number
86753205
Registration Number
5172332
Status
700 - Registered
Status Date
2017-03-28
Filing Date
2015-09-10
Registration Number
5172332
Registration Date
2017-03-28
Mark Drawing
2000 - Illustration: Drawing or design without any word(s)/letter(s)/ number(s)
Typeset
Design Searches
260109, 260111, 260113, 260116, 260127, 260128, 260131, 261509, 261516 - Geometric figures, objects, humans, plants or animals forming or bordering the perimeter of a circle. Letters, numerals, punctuation, geometric figures, objects, humans, plants or animals comprising a circle. Two circles. Circles touching or intersecting. Circles containing irregular exterior lining or elements not amounting to a decorative border. Miscellaneous circular designs with an irregular circumference. Five or more circles. Polygons made of geometric figures, objects, humans, plants or animals. Polygons touching or intersecting.
Published for Opposition Date
2016-06-21
Attorney Name
Law Office Assigned Location Code
N30
Employee Name
BROWN, TRICIA LYNN
Statements
Indication of Colors claimed
Color is not claimed as a feature of the mark.
Description of Mark
The mark consists of 11 circles connected by single lines forming 2 connected polygons.
Goods and Services
Business consulting and management in the field of clinical trials, namely, clinical data and regulatory submission management on behalf of medical, biopharmaceutical and biotechnology companies to assist them with clinical research, clinical trials and applications for drug approval; regulatory submission management, namely, assisting others in preparing and filing applications for new medical devices, medical technologies, medications, biopharmaceuticals, and biotechnology with governmental regulatory bodies around the world; regulatory submission management, namely, providing simultaneous multiple country submissions of applications for the registration and approval of medical devices, medical technologies, medications, biopharmaceuticals, and biotechnology; providing information online regarding regulatory submission management; business management consulting, strategic planning and business advisory services provided to medical, medical technology, biopharmaceutical, and biotechnology companies, and government agencies, educational and research institutions, law firms, and investment firms in the fields of biologics, pharmaceuticals and medical devices; providing consulting services in the field of regulatory submission management to medical companies to assist them with applications for drug and medical device approval; regulatory submission management, namely, assisting others in preparing and filing applications for new drugs and medical devices with governmental regulatory bodies; business consulting services in the field of biologics, pharmaceuticals, prescription drugs and medical devices
Goods and Services
Consulting services for others in the field of designing pre-clinical and clinical studies and trials for others; product research and development for others in the fields of biologics, pharmaceuticals, prescription drugs and medical devices; quality management services, namely, quality evaluation and analysis, quality assurance and quality control in the field of biologics, pharmaceuticals, prescription drugs and medical devices; scientific consulting and research services relating to the field of pharmaceuticals, medical devices, and life sciences, namely, biology, and medicine; consulting services in the fields of biotechnology and pharmaceuticals, namely, design, development, technical verification and technical validation of manufacturing and filling processes, drug formulations, testing protocols and analytical methods; and scientific and technological services, namely, scientific research, analysis, testing, and related scientific studies in the field of chemistry and molecular biology, namely, biologics and protein detection, characterization, analysis and testing; consulting services in the field of medical device product approval for commercial purposes
Goods and Services
Regulatory compliance consulting services, namely, reviewing standards and practices to assist clients with compliance with governmental laws, regulations and rules in the fields of medical devices, medical technologies, medications, biopharmaceuticals, and biotechnology; providing technical regulatory affair consulting services directed to medical, medical technology, biopharmaceutical, and biotechnology companies in order to assure compliance with food and drug administration's laws and regulations; provision of information online pertaining to regulatory compliance in the fields of medical devices, medical technologies, medications, biopharmaceuticals, and biotechnology; and regulatory compliance consulting in the fields of biologics, pharmaceuticals, prescription drugs and medical devices
Classification Information
International Class
035 - Advertising; business management; business administration; office functions. - Advertising; business management; business administration; office functions.
US Class Codes
100, 101, 102
Class Status Code
6 - Active
Class Status Date
2015-09-15
Primary Code
035
First Use Anywhere Date
2016-01-15
First Use In Commerce Date
2016-01-15
International Class
042 - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services. - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services.
US Class Codes
100, 101
Class Status Code
6 - Active
Class Status Date
2015-09-15
Primary Code
042
First Use Anywhere Date
2016-01-15
First Use In Commerce Date
2016-01-15
International Class
045 - Personal and social services rendered by others to meet the needs of individuals; security services for the protection of property and individuals. - Personal and social services rendered by others to meet the needs of individuals; security services for the protection of property and individuals.
US Class Codes
100, 101
Class Status Code
6 - Active
Class Status Date
2015-09-15
Primary Code
045
First Use Anywhere Date
2016-01-15
First Use In Commerce Date
2016-01-15
Current Trademark Owners
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Trademark Owner History
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
10 - Original Applicant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Correspondences
Name
DONNA M.D. THOMAS
Address
Please log in with your Justia account to see this address.
Prior Registrations
| Relationship Type | Reel Number |
| Prior Registration | 2975687 |
Trademark Events
| Event Date | Event Description |
| 2015-09-14 | NEW APPLICATION ENTERED IN TRAM |
| 2015-09-15 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2015-09-16 | NOTICE OF DESIGN SEARCH CODE E-MAILED |
| 2015-12-22 | ASSIGNED TO EXAMINER |
| 2016-01-05 | NON-FINAL ACTION WRITTEN |
| 2016-01-05 | NON-FINAL ACTION E-MAILED |
| 2016-01-05 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2016-05-18 | EXAMINERS AMENDMENT -WRITTEN |
| 2016-05-18 | EXAMINERS AMENDMENT E-MAILED |
| 2016-05-18 | NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED |
| 2016-05-18 | EXAMINER'S AMENDMENT ENTERED |
| 2016-05-18 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2016-06-01 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2016-06-21 | PUBLISHED FOR OPPOSITION |
| 2016-06-21 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2016-08-16 | NOA E-MAILED - SOU REQUIRED FROM APPLICANT |
| 2017-01-16 | TEAS STATEMENT OF USE RECEIVED |
| 2017-02-03 | CASE ASSIGNED TO INTENT TO USE PARALEGAL |
| 2017-01-16 | USE AMENDMENT FILED |
| 2017-02-03 | STATEMENT OF USE PROCESSING COMPLETE |
| 2017-02-21 | ALLOWED PRINCIPAL REGISTER - SOU ACCEPTED |
| 2017-02-22 | NOTICE OF ACCEPTANCE OF STATEMENT OF USE E-MAILED |
| 2017-03-28 | REGISTERED-PRINCIPAL REGISTER |