SANOVEL - Trademark Details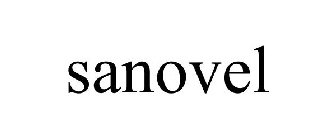
Status: 710 - Cancelled - Section 8
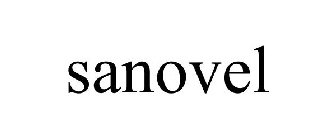
Serial Number
85749420
Registration Number
4438655
Word Mark
SANOVEL
Status
710 - Cancelled - Section 8
Status Date
2020-07-03
Filing Date
2012-10-09
Registration Number
4438655
Registration Date
2013-11-26
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2013-09-10
Attorney Name
Law Office Assigned Location Code
M50
Employee Name
KEAM, ALEX S
Statements
Indication of Colors claimed
Color is not claimed as a feature of the mark.
Goods and Services
pharmaceutical preparations for the treatment of cardiovascular disorders, skeleto-muscular disorders, general infections, viral infections, central nervous system disorders, gastro-intestinal disorders, fungal diseases, erectile dysfunction, respiratory diseases, allergy, ophthalmic diseases, containing one or more of the following active ingredients - cromolin sodium, amlodipin besylate, alendronate sodium, chlorazepate dipotassium, atropine sulphate, atorvastatin calcium, candesartan cilexetil, ceftriaxone sodium, ephedrine hcl, guaiphenesine, zafirlukast, donepezil hcl, diflunisal, dipyridamole, famotidine, losartan potassium, hydro-chlorothiazide, meloxicam, phenyleprine hcl, fexofenadine hcl, indapamide, sulfisoxazole, clopidogrel, lansoprazole, clarithromycin, flurbiprofen, nimesulide, pantoprazole, paracetamole, pilocarpine hcl, lisinopril, pseudoephedrine hcl, ciprofloxacin hcl, sertraline hcl, cyclopentolate hcl, dihydroergotoxine methansulfonate, terbinafine, timolol maleate, tiamphenicol, azithromycin, tenoxicam, fluconazole, digoxine, tioconazole, dimenhydrinate, quinidine arabogalactane sulphate, quinidine phenylethyl barbiturate, diltiazem hcl, buspirone hcl, amiodarone hcl, trazodone, difluprednate, enoxacin, lactulose, ketorolac, pentoxifylline, vitamin e, chlorhexidine, cetrimide, propyphenazone, caffeine, cetirizine, phenylpropanolamine, zolpidem, bacampicillin hcl, roxithromycin, sultamicillin tosilate, acetylsalicylic acid, ampicillin na, sulbactam na, sildenafil citrate, ambroxol hcl, ribavirin, loratadine, etodolac, omeprazole, oxybutynin chloride, quinidine sulphate, olanzapine, raloxifen, pioglitazone, moxifloxacin, rosiglitazone, sibutramin, carvedilol, montelukast, citalopram, gliclazide, cefaclor, ezetimibe, rofecoxib, quetiapine fumarate, metformin hcl, acarbose, chlorpheniramine maleate, nateglinide, leflunomide, valsartan, cefazolin sodium, diacerein, paroxetine, levofloxacin, levetiracetam, zolmitriptan, glibenclamide, tizanidine, perindopril erbumine, ramipril, ceftazidime, cefuroxime axetyl, thiocolchicoside, acamprosate, acetylcysteine, alpha calcidol, alpha lipoic acid, aripiprazole, atomoxetine, balsalazide disodium, butenafine hcl, calcitriol, carbonyl iron, folic acid, calcium carbonate, magnesium hydroxide, cefdinir, cefepime hcl, cefixime, cefpodoxime proxetil, cefprozil, cephalexine monohydrate, ciclesonide, cilazapril, cilostazol, cinitapride, citicoline, co-enzyme q10, darifenasin, deferiprone, dothiepin, duloxetine, eletriptan, escitalopram, esomeprazole, etoricoxib, felodipine, fenofibrate, ferrous sulphate, zinc sulphate, fluvastatin, frovatriptan, gabapentin, galantamine, gemifloxacin, ginkgo biloba, glimepride, granisetron, ibandronate, irbesartan, iron hydroxide polymaltose, lamivudine, lamotrigine, levetiracetam, letrozole, linezolide, memantine hcl, metoprolol, midodrine, mirtazapine, mizolastine, mosapride citrate dihydrate, mycophenolate mofetil, nebivolol, nefazodone, niacin, nitazoxanide, ondansetron, ornidazole, oxcarbazepine, pimecrolimus, pravastatin, pregabalin, rabeprazol, rebamipide, repaglinide, risedronate, risperidone, rivastigmine, rosuvastatin, saccharomyces boulardii, tacrolimus, tadalafil, tamsulosin hcl, tazaroten, tazobactam sodium, piperacillin sodium, tegaserod, telithromycin, terazocin, teriparatide, tolterodine tartrate, topiramate, trandolapril, trimethazidine, valacyclovir, valproate sodium, venlafaxine, voriconazole, ximelagatran, zaleplon, ziprasidone, zoledronic acid
Classification Information
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
2 - Sec. 8 - Entire Registration
Class Status Date
2020-07-03
Primary Code
005
Correspondences
Name
Keith E. Danish
Address
Please log in with your Justia account to see this address.
Prior Registrations
| Relationship Type | Reel Number |
| Prior Registration | 3154206 |
| Prior Registration | 3202340 |
Foreign Application Information
| Filing Date | Application Number | Country | Foreign Priority Claim In |
| Turkey | False |
Trademark Events
| Event Date | Event Description |
| 2012-10-12 | NEW APPLICATION ENTERED IN TRAM |
| 2012-10-16 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2012-12-11 | TEAS CHANGE OF CORRESPONDENCE RECEIVED |
| 2013-02-06 | ASSIGNED TO EXAMINER |
| 2013-02-06 | EXAMINERS AMENDMENT AND/OR PRIORITY ACTION - COMPLETED |
| 2013-02-06 | EXAMINER'S AMENDMENT/PRIORITY ACTION E-MAILED |
| 2013-02-06 | NOTIFICATION OF EXAMINER'S AMENDMENT/PRIORITY ACTION E-MAILED |
| 2013-02-06 | ASSIGNED TO LIE |
| 2013-02-07 | COMBINED EXAMINER'S AMENDMENT/PRIORITY ACTION ENTERED |
| 2013-08-01 | PAPER RECEIVED |
| 2013-08-06 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2013-08-06 | AMENDMENT FROM APPLICANT ENTERED |
| 2013-08-06 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2013-08-07 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2013-08-21 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2013-09-10 | PUBLISHED FOR OPPOSITION |
| 2013-09-10 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2013-11-26 | REGISTERED-PRINCIPAL REGISTER |
| 2018-11-26 | COURTESY REMINDER - SEC. 8 (6-YR) E-MAILED |
| 2020-07-03 | CANCELLED SEC. 8 (6-YR) |