SYMPHOMAX - Trademark Details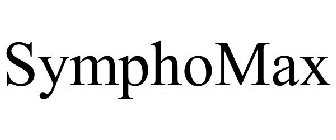
Status: 602 - Abandoned-Failure To Respond Or Late Response
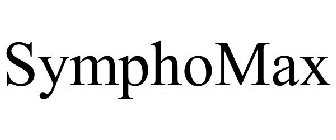
Serial Number
85193730
Word Mark
SYMPHOMAX
Status
602 - Abandoned-Failure To Respond Or Late Response
Status Date
2013-03-22
Filing Date
2010-12-08
Mark Drawing
4000 - Standard character mark
Typeset
Attorney Name
Law Office Assigned Location Code
L40
Employee Name
BOONE, JOHN C
Statements
Goods and Services
Host cells for medical or clinical use; cells that manufacture antibodies, fragments of antibodies or derivatives of antibodies for medical or clinical use; transfected cells that manufacture antibodies, fragments of antibodies or derivatives of antibodies for medical or clinical use; pharmaceuticals, namely, central nervous system depressants, preparations for ophthalmic use, anti-histaminics, cardiotonics, arrhythmia treating agents, diuretics, hypotensors, prophylactic for the treatment of asthma, antitussives and expectorants, stomachics and digestives, antacids, hormones, namely, pituitary hormones for the treatment of infertility, thyroid hormones for the treatment of hyperthyroidism, anabolic hormones for the treatment of aplastic anemia and osteoporosis, corticosteroids for treatment of hypopituitarism, congenital adrenal hyperplasia, rheumatoid arthritis, rheumatic fever, crohn's disease, and chronic active hepatitis, estrogen and progesterone used as combined oral contraceptives, urinary antiseptics, analgesics, anti-itching agents, cataplasms for the treatment of bruises, lumbago and stiff shoulder, pharmaceutical preparations used to treat hair disorders, vitamin preparations, food enriching agents for treating vitamin deficiencies, anti-coagulants, enzyme preparations for control of malabsorption and diarrhea, anti-cancer preparations, anti-sarcoma preparations, antibiotics and their preparations for the treatment of infectious diseases, antituberculotic agents, influenza vaccines, diphtheria vaccines, tetanus vaccines, gas gangrene vaccines, Japanese encephalitis vaccines, in vitro tetrodotoxines for medical use, antiprotozoals for the treatment of trichomoniasis and dermatophytosis, anthelmintics for the treatment of threadworm and toxoplasmaosis, naturally occurring drugs for treating peptic ulcers, charred drugs for treating peptic ulcers, moxa for treating peptic ulcers, veterinary drugs for the treatment of distemper in dogs, distomatosis in dogs, coccidian in chickens, and dysentery in pigs, germination controllers and plant rearing agents, namely, herbicides, fungicides, insecticides, bactericides and rodenticides for domestic and or agricultural use, antiseptics, drugs for arteriosclerosis, oncostatic drugs for the treatment for cancer, multienzyme digestants for in vivo use; antifungal antibiotics for treating trichomoniasis and dermatophytosis, anti parkinsonism drugs, minor tranquilizers, and anti anxiety agents, medicinal preparations for the treatment of the central nervous system, the respiratory tract and the cardiovascular system, anti-bacterial preparations, monolithic preparations, medicinal preparations for the treatment of peptic ulcers, anti-inflammatory preparations, analgesic preparations, cephalosporin, cocarboxylase preparations, glutathione preparations, preparations for improving the metabolism and the peripheral circulation, plasma substitutes and extracorporeal circulation flow improvement preparations, diuretic and anti-hypertensive preparations, in vitro diagnostic reagents for medical use, in vitro extra corporeal diagnostic reagents for medical use, in vitro x-ray contrast agents for medical use, in vitro diagnostic media, pharmaceutical preparations for the treatment of insomnia and other sleep related diseases
Goods and Services
Contract manufacturing service in the field of antibodies, fragments of antibodies, and derivatives of antibodies; contract manufacturing service in the field of cells that manufacture antibodies, fragments of antibodies, and derivatives of antibodies; contract manufacturing service in the field of transfected cells that manufacture antibodies, fragments of antibodies, and derivatives of antibodies; contract manufacturing service in the field of host cells; contract extracting, refining, synthesizing, manufacturing and preparing pharmaceuticals, agricultural chemicals, and chemicals; providing information in the field of manufacturing technology in the field of antibodies, fragments of antibodies, and derivatives of antibodies; technical consultation in the field of manufacturing technology in the field of antibodies, fragments of antibodies, and derivatives of antibodies; providing information in the field of manufacturing technology in the field of cells that manufacture antibodies, fragments of antibodies, and derivatives of antibodies; technical consultation in the field of manufacturing technology in the field of cells that manufacture antibodies, fragments of antibodies, and derivatives of antibodies; providing information in the field of manufacturing technology in the field of transfected cells that manufacture antibodies, fragments of antibodies, and derivatives of antibodies; technical consultation in the field of manufacturing technology in the field of transfected cells that manufacture antibodies, fragments of antibodies, and derivatives of antibodies; providing information in the field of manufacturing technology in the field of host cells; technical consultation in the field of manufacturing technology in the field of host cells; providing technical information in the field of extracting, refining, synthesizing, manufacturing and preparing pharmaceuticals, agricultural chemicals, and chemicals technical consultation in the field of extracting, refining, synthesizing, manufacturing and preparing pharmaceuticals, agricultural chemicals, and chemicals
Goods and Services
Development of new products for others in the field of antibodies, fragments of antibodies, derivatives of antibodies, cells that manufacture antibodies, fragments of antibodies, and derivatives of antibodies, transfected cells that manufacture antibodies, fragments of antibodies, and derivatives of antibodies, and host cells; providing technical information in the field of development of new products in the field of antibodies, fragments of antibodies, derivatives of antibodies, cells that manufacture antibodies, fragments of antibodies, and derivatives of antibodies, transfected cells that manufacture antibodies, fragments of antibodies, and derivatives of antibodies, and host cells; technical consultation in the field of development of new products in the field of antibodies, fragments of antibodies, derivatives of antibodies, cells that manufacture antibodies, fragments of antibodies, and derivatives of antibodies, transfected cells that manufacture antibodies, fragments of antibodies, and derivatives of antibodies, and host cells
Pseudo Mark
SYMPHONY MAX
Classification Information
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
6 - Active
Class Status Date
2010-12-13
Primary Code
005
International Class
040 - Treatment of materials. - Treatment of materials.
US Class Codes
100, 103, 106
Class Status Code
6 - Active
Class Status Date
2011-12-07
Primary Code
040
International Class
042 - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services. - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services.
US Class Codes
100, 101
Class Status Code
6 - Active
Class Status Date
2011-12-07
Primary Code
042
Correspondences
Name
KAUSHAL R. ODEDRA
Address
Please log in with your Justia account to see this address.
Foreign Application Information
| Filing Date | Application Number | Country | Foreign Priority Claim In |
| 2010-12-08 | 2010-095220 | Japan | True |
| 0000-00-00 | Japan | False |
Trademark Events
| Event Date | Event Description |
| 2010-12-11 | NEW APPLICATION ENTERED IN TRAM |
| 2010-12-13 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2010-12-14 | NOTICE OF PSEUDO MARK MAILED |
| 2011-03-12 | ASSIGNED TO EXAMINER |
| 2011-03-21 | NON-FINAL ACTION WRITTEN |
| 2011-03-21 | NON-FINAL ACTION MAILED |
| 2011-09-20 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2011-09-29 | ASSIGNED TO LIE |
| 2011-10-07 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2011-10-07 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2011-11-15 | SUSPENSION LETTER WRITTEN |
| 2011-11-16 | LETTER OF SUSPENSION MAILED |
| 2011-11-22 | TEAS RESPONSE TO SUSPENSION INQUIRY RECEIVED |
| 2011-11-22 | TEAS RESPONSE TO SUSPENSION INQUIRY RECEIVED |
| 2011-11-22 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2011-11-23 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2011-12-24 | NON-FINAL ACTION WRITTEN |
| 2011-12-27 | NON-FINAL ACTION MAILED |
| 2012-04-09 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2012-04-09 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2012-04-10 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2012-08-08 | ASSIGNED TO EXAMINER |
| 2012-08-09 | ASSIGNED TO EXAMINER |
| 2012-08-24 | NON-FINAL ACTION WRITTEN |
| 2012-08-24 | NON-FINAL ACTION MAILED |
| 2013-03-22 | ABANDONMENT - FAILURE TO RESPOND OR LATE RESPONSE |
| 2013-03-22 | ABANDONMENT NOTICE MAILED - FAILURE TO RESPOND |