ADILNA - Trademark Details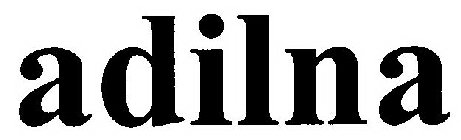
Status: 404 - U.S. registration cancelled because International Registration cancelled in whole or in part
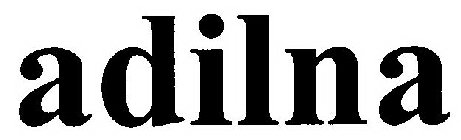
Serial Number
79016579
Registration Number
3204166
Word Mark
ADILNA
Status
404 - U.S. registration cancelled because International Registration cancelled in whole or in part
Status Date
2015-07-12
Filing Date
2005-07-11
Registration Number
3204166
Registration Date
2007-01-30
Mark Drawing
5000 - Illustration: Drawing with word(s)/letter(s)/number(s) in Stylized form
Typeset
Published for Opposition Date
2006-11-14
Law Office Assigned Location Code
M60
Employee Name
EDWARDS, ALICIA COLLIN
Statements
Goods and Services
PHARMACEUTICAL PREPARATIONS FOR THE TREATMENT OF CARDIOVASCULAR DISORDERS, SKELETO-MUSCULAR DISORDERS, GENERAL INFECTIONS, VIRAL INFECTIONS, CENTRAL NERVOUS SYSTEM DISORDERS, GASTRO-INTESTINAL DISORDERS, FUNGAL DISEASES, ERECTILE DYSFUNCTION, RESPIRATORY DISEASES, ALLERGY, OPHTHALMIC DISEASES, CONTAINING ONE OR MORE OF THE FOLLOWING ACTIVE INGREDIENTS - CROMOLIN SODIUM, AMLODIPIN BESYLATE, ALENDRONATE SODIUM, CHLORAZEPATE DIPOTASSIUM, ATROPINE SULPHATE, ATORVASTATIN CALCIUM, CANUESARTAN CILEXETIL, CEFTRIAXONE SODIUM, EPHEDRINE HCL, GUAIPHENESINE, ZAFIRLUKAST, DONEPEZIL HCL, DIFLUNISAL, DIPYRIDAMOLE, FAMOTIDINE, LOSARTAN POTASSIUM, HYDRO CHLOROTHIAZIDE, MELOXICAM, PHENYLEPRINE HCL, FEXOFENADINE HCL, INDAPAMIDE, SUIJFISOXAZOLE, CLOPIDOGREL, LANSOPRAZOLE, CLARITHROMYCIN, FLURBIPROFEN, NIMESULIDE, PANTOPRAZOLE, PARACETAMOLE, PILOCARPINE HCL, LISINOPRIL, PSEUDOEPHEDRINE HCL, CIPROFLOXACIN HCL, SERTRALINE HCL, CYCLOPENTOLATE HCL, DIHYDROERGOTOXINE METHANSULFONATE, TERBINAFINE, TIMOLOL MALEATE, TIAMPHENICOL, AZITHROMYCIN, TENOXICAM, FLUCONAZOLE, DIGOXINE, TIOCONAZOLE, DIMENHYDRINATE, QUINIDINE ARABOGALACTANE SULPHATE, QUINIDINE PHENYIJETHYL BARBITURATE, DILTIAZEM HCL, BUSPIRONE HCL, AMIODARONE HCL, TRAZODONE, DIFLUPREDNATE, ENOXACIN, LACTULOSE, KETOROLAC, PENTOXIFYLLINE, VITAMIN E, CHLORHEXIDINE, CETRIMIDE, PROPYPHENAZONE, CAFFEINE, CETIRIZINE, PHENYLPROPANOLAMINE, ZOLPIDEM, BACAMPICILLIN HCL, ROXITHROMYCIN, SULTAMICILLIN TOSILATE, ACETYLSALICYLIC ACID, AMPICILLIN NA, SULBACTAM NA, SILDENAFIL CITRATE, AMBROXOL HCL, RIBAVIRIN, LORATADINE, ETODOLAC, OMEPRAZOLE, OXYBUTYNIN CHLORIDE, QUINIDINE SULPHATE, OLANZAPINE, RALOXIFEN, PIOGLITAZONE, MOXIFLOXACIN, ROSIGLITAZONE, SIBUTRAMIN, CARVEDILOL, MONTELUKAST, CITALOPRAM, GLICLAZIDE, CEFACLOR, EZETIMIBE, ROFECOXIB, QUETIAPINE FUMARATE, METFORMIN HCL, ACARBOSE, CHLORPHENIRAMINE MALEATE, NATEGLINIDE, LEFLUNOMIDE, VALSARTAN, CEFAZOLIN SODIUM, DIACEREIN, PAROXETINE, LEVOFLOXACIN, LEVETIRACETAM, ZOLMITRIPTAN, GLIBENCLAMIDE, TIZANIDINE, PERINDOPRIL ERBUMINE, RAMIPRIL, CEFTAZIDIME, CEFUROXIME AXETYL, THIOCOLCHICOSIDE, ACAMPROSATE, ACETYLCYSTEINE, ALPHA CALCIDOL, ALPHA LIPOIC ACID, ARIPIPRAZOLE, ATOMOXETINE, BALSALAZIDE DISODIUM, BUTENAFINE HCL, CALCITRIOL, CARBONYL IRON, FOLIC ACID, CALCIUM CARBONATE, MAGNESIUM HYDROXIDE, CEFDINIR, CEFEPIME HCL, CEFIXIME, CEFPODOXIME PROXETIL, CEPPROZIL, CEPHALEXINE MONOHYDRATE, CICLESONIDE, CILAZAPRIL, CILOSTAZOL, CINITAPRIDE, CITICOLINE, CO-ENZYME Q10, DARIFENASIN, DEFERIPRONE, DOTHIEPIN, DULOXETINE, ELETRIPTAN, ESCITALOPRAM, ESOMEPRAZOLE, ETORICOXIB, FELODIPINE, FENOFIBRATE, FERROUS SULPHATE, ZINC SULPHATE, FLUVASTATIN, FROVATRIPTAN, GABAPENTIN, GALANTAMINE, GEMIFLOXACIN, GINKGO BILOBA, GLIMEPRIDE, GRANISETRON, IBANDRONATE, IRBESARTAN, IRON HYDROXIDE POLYMALTOSE, LAMIVUDINE, LAMOTRIGINE, LEVETIRACETAM, LETROZOLE, LINEZOLIDE, MEMANTINE HCL, METOPROLOL, MIDODRINE, MIRTAZAPINE, MIZOLASTINE, MOSAPRIDE CITRATE DIHYDRATE, MYCOPHENOLATE MOFETIL, NEBIVOLOL, NEFAZODONE, NIACIN, NITAZOXANIDE, ONDANSETRON, ORNIDAZOLE, OXCARBAZEPINE, PIMECROLIMTJS, PRAVASTATIN, PREGABALIN, RABEPRAZOL, REBAMIPIDE, REPAGLINIDE, RISEDRONATE, RISPERIDONE, RIVASTIGMINE, ROSUVASTATIN, SACCHAROMYCES BOULARDII, TACROLIMUS, TADALAFIL, TAMSULOSIN HCL, TAZAROTEN, TAZOBACTAM SODIUM, PIPERACILLIN SODIUM, TEGASEROD, TELITHROMYCIN, TERAZOCIN, TERIPARATIDE, TOLTERODINE TARTRATE, TOPIRAMATE, TRANDOLAPRIL, TRIMETHAZIDINE, VALACYCLOVIR, VALPROATE SODIUM, VENLAFAXINE, VORICONAZOLE, XIMELAGATRAN, ZALEPLON, ZIPRASIDONE, LEDRONIC ACID
Classification Information
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
F - Section 71 - Cancelled
Class Status Date
2013-09-27
Primary Code
005
Correspondences
Name
SANOVEL ILAÇ
Address
Please log in with your Justia account to see this address.
International Registrations
International Registration Number
0865146
International Registration Date
2005-07-11
International Publication Date
2005-12-01
International Renewal Date
2015-07-11
Auto Protection Date
2007-05-10
International Status
102 - Death of IR (Dead as a Result of Non-Renewal, Renunciation or Cancellation by Holder of International Registration)
International Status Date
2015-07-12
Priority Claimed In
False
First Refusal In
True
Trademark Events
| Event Date | Event Description |
| 2005-11-10 | SN ASSIGNED FOR SECT 66A APPL FROM IB |
| 2005-11-14 | NEW APPLICATION ENTERED IN TRAM |
| 2006-01-30 | ASSIGNED TO EXAMINER |
| 2006-01-30 | NON-FINAL ACTION WRITTEN |
| 2006-01-31 | NON-FINAL ACTION (IB REFUSAL) PREPARED FOR REVIEW |
| 2006-01-31 | NON-FINAL ACTION MAILED - REFUSAL SENT TO IB |
| 2006-02-17 | REFUSAL PROCESSED BY IB |
| 2006-07-14 | TEAS CHANGE OF CORRESPONDENCE RECEIVED |
| 2006-07-24 | PAPER RECEIVED |
| 2006-07-24 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2006-08-17 | AMENDMENT FROM APPLICANT ENTERED |
| 2006-09-04 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2006-09-15 | ASSIGNED TO LIE |
| 2006-09-22 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2006-10-25 | NOTICE OF PUBLICATION |
| 2006-11-14 | PUBLISHED FOR OPPOSITION |
| 2007-01-30 | REGISTERED-PRINCIPAL REGISTER |
| 2007-08-21 | FINAL DISPOSITION NOTICE CREATED, TO BE SENT TO IB |
| 2007-08-27 | FINAL DISPOSITION PROCESSED |
| 2007-08-27 | FINAL DISPOSITION NOTICE SENT TO IB |
| 2008-05-23 | FINAL DECISION TRANSACTION PROCESSED BY IB |
| 2009-07-02 | CHANGE OF OWNER RECEIVED FROM IB |
| 2012-11-03 | CHANGE OF NAME/ADDRESS REC'D FROM IB |
| 2013-09-27 | CANCELLED SECTION 71 |
| 2014-05-27 | TOTAL INVALIDATION OF REG EXT PROTECTION CREATED |
| 2014-11-06 | GENERIC MADRID TRANSACTION CREATED |
| 2014-11-06 | GENERIC MADRID TRANSACTION SENT TO IB |
| 2015-06-05 | TOTAL INVALIDATION PROCESSED BY THE IB |
| 2016-01-31 | DEATH OF INTERNATIONAL REGISTRATION |
| 2016-01-31 | NOTIFICATION OF EFFECT OF CANCELLATION OF INTL REG MAILED |