SERACARE - Trademark Details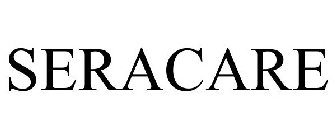
Status: 702 - Section 8 & 15-Accepted And Acknowledged
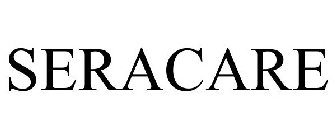
Serial Number
77832375
Registration Number
4451588
Word Mark
SERACARE
Status
702 - Section 8 & 15-Accepted And Acknowledged
Status Date
2020-08-10
Filing Date
2009-09-22
Registration Number
4451588
Registration Date
2013-12-17
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2011-08-30
Attorney Name
Law Office Assigned Location Code
L30
Employee Name
JUN, WON KYUNG WENDY
Statements
Indication of Colors claimed
Color is not claimed as a feature of the mark.
Goods and Services
Diagnostic chemicals, reagents or preparations for scientific, research or manufacturing use in chemical, pharmaceutical and biotechnology industries; Diagnostic reagents for clinical, medical laboratory or manufacturing use in chemical, pharmaceutical and biotechnology industries; Diagnostic reagents for in vitro use in biochemistry, clinical chemistry, microbiology; Reagents and tools, namely, animal serums, bovine serum albumin, and human blood products, namely, plasma, serum, cells, and human serum albumin, and chromogenic substrates, all for research purposes or manufacturing use in chemical, pharmaceutical and biotechnology industries; Chemical reagents other than for medical or veterinary purposes; Chemical preparations for scientific purposes; Chemical ingredients for use as exipients or diluents in the manufacture of pharmaceuticals; Chemical reagents for reference control, namely, genetic testing controls and controls for the testing of human serum, infectious disease antibodies and antigens, and viral nucleic acids, all for industrial use and not for medical purposes; Chemical solutions in the nature of quality control standard solutions for testing human serum, infectious disease antibodies and antigens, human genetics, and viral nucleic acids, all for industrial use and not for medical purposes; Chemical control serum for scientific or research use in the nature of quality controls for infectious disease and genetic testing; Biochemical control serum for scientific or research use in the nature of quality controls for infectious disease and genetic testing; Biochemical standard serum for scientific or research use; Quality control standard solutions and quality control materials for testing and calibrating medical apparatus and reagents for scientific and research purposes and for clinical or medical laboratory use; Standard serum, control serum, biochemical standard serum, biochemical control serum for scientific and research purposes and for clinical or medical laboratory use
Goods and Services
Diagnostic reagents for medical and veterinary use; Clinical medical reagents; Diagnostic chemicals, reagents or preparations for medical and veterinary use; Chemical reagents for medical and veterinary use; Chemical reagents for pharmaceutical purposes and use in the pharmaceutical industry, namely, chemical reagents for use as diluents and excipients; Testing reagents for medical or clinical use; Genetic testing controls for the testing of human serum, infectious disease antibodies and antigens, and viral nucleic acids, namely, medical diagnostic reagents and assays; Chemical reference materials, namely, quality controls and panels in the nature of human compounds for medical or veterinary use; Chemical reference specimens, namely, serological, viral, genetic and nucleic acid reference specimens in the nature of human serum, infectious disease antibodies and antigens for medical or veterinary use
Goods and Services
Storage of biospecimens
Goods and Services
Cryogenic preservation of biospecimens
Goods and Services
Training services in the field of biospecimen processing and management
Goods and Services
Custom design and development of chemical reagents and biochemical assays; Providing reagent sample testing for others in the fields of, science and research related thereto; Research support services in the nature of assay development and validation, product development, and report generation, all in the fields of virology, immunological, biological and biochemical research
Pseudo Mark
SERA CARE
Translation of Words in Mark
The wording "SERA" has no meaning in a foreign language.
Classification Information
International Class
001 - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry; unprocessed artificial resins, unprocessed plastics; manures; fire extinguishing compositions; tempering and soldering preparations; chemical substances for preserving foodstuffs; tanning substances; adhesives used in industry. - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry; unprocessed artificial resins, unprocessed plastics; manures; fire extinguishing compositions; tempering and soldering preparations; chemical substances for preserving foodstuffs; tanning substances; adhesives used in industry.
US Class Codes
001, 005, 006, 010, 026, 046
Class Status Code
6 - Active
Class Status Date
2009-09-25
Primary Code
001
First Use Anywhere Date
2001-09-26
First Use In Commerce Date
2001-09-26
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
6 - Active
Class Status Date
2009-09-25
Primary Code
005
First Use Anywhere Date
2001-09-26
First Use In Commerce Date
2001-09-26
International Class
042 - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services. - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services.
US Class Codes
100, 101
Class Status Code
6 - Active
Class Status Date
2009-09-25
Primary Code
042
First Use Anywhere Date
2001-09-26
First Use In Commerce Date
2001-09-26
Current Trademark Owners
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Trademark Owner History
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
10 - Original Applicant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Correspondences
Name
Emilia F. Cannella
Address
Please log in with your Justia account to see this address.
Madrid International Filings
Entry Number
1
Reference Number
A0019152
Original Filing Date USPTO
2010-03-22
International Registration Number
1052206
International Registration Date
2010-03-22
International Status Code
480
International Renewal Date
2030-03-22
| Madrid History Events | ||
| Date | Code | Description |
| 2014-04-28 | CBMPP | CEASING OF EFFECT PROCESSED |
| 2010-10-22 | CREAT | APPLICATION FOR IR REGISTERED BY IB |
| 2014-05-01 | CBPSP | PARTIAL CEASING OF EFFECT NOTICE SENT TO IB |
| 2014-03-17 | CBPCP | PARTIAL CEASING OF EFFECT TO BE PROCESSED |
| 2020-04-16 | RENWL | INTERNATIONAL REGISTRATION RENEWED |
| 2010-03-22 | NEWAP | NEW APPLICATION FOR IR RECEIVED |
| 2010-03-22 | ACERT | AUTOMATICALLY CERTIFIED |
| 2010-03-22 | APPST | IR CERTIFIED AND SENT TO IB |
| 2010-04-23 | IRREQ | IRREGULARITY NOTICE RECEIVED FROM IB (RESPONSE REQUIRED) |
| 2010-07-16 | IRRCV | IRREGULARITY RESPONSE RECEIVED FROM APPLICANT |
| 2010-07-21 | IRRRJ | RESPONSE TO IRREGULARITY REVIEWED AND REJECTED |
Trademark Events
| Event Date | Event Description |
| 2009-09-25 | NEW APPLICATION ENTERED IN TRAM |
| 2009-09-25 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2009-09-26 | NOTICE OF PSEUDO MARK MAILED |
| 2009-12-18 | ASSIGNED TO EXAMINER |
| 2009-12-19 | NON-FINAL ACTION WRITTEN |
| 2009-12-19 | NON-FINAL ACTION E-MAILED |
| 2009-12-19 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2009-12-19 | NON-FINAL ACTION WRITTEN |
| 2009-12-19 | NON-FINAL ACTION E-MAILED |
| 2009-12-19 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2010-06-21 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2010-07-09 | ASSIGNED TO LIE |
| 2010-07-09 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2010-07-09 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2010-07-29 | FINAL REFUSAL WRITTEN |
| 2010-07-29 | FINAL REFUSAL E-MAILED |
| 2010-07-29 | NOTIFICATION OF FINAL REFUSAL EMAILED |
| 2010-10-13 | TEAS CHANGE OF CORRESPONDENCE RECEIVED |
| 2011-01-31 | EXAMINERS AMENDMENT AND/OR PRIORITY ACTION - COMPLETED |
| 2011-01-31 | EXAMINER'S AMENDMENT/PRIORITY ACTION E-MAILED |
| 2011-01-31 | NOTIFICATION OF EXAMINER'S AMENDMENT/PRIORITY ACTION E-MAILED |
| 2011-02-01 | COMBINED EXAMINER'S AMENDMENT/PRIORITY ACTION ENTERED |
| 2011-02-01 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2011-02-03 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2011-02-03 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2011-02-17 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2011-02-17 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2011-02-28 | WITHDRAWN FROM PUB - OG REVIEW QUERY |
| 2011-03-15 | PREVIOUS ALLOWANCE COUNT WITHDRAWN |
| 2011-03-23 | NON-FINAL ACTION WRITTEN |
| 2011-03-23 | NON-FINAL ACTION E-MAILED |
| 2011-03-23 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2011-07-18 | EXAMINERS AMENDMENT -WRITTEN |
| 2011-07-18 | EXAMINERS AMENDMENT E-MAILED |
| 2011-07-18 | NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED |
| 2011-07-19 | EXAMINER'S AMENDMENT ENTERED |
| 2011-07-20 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2011-07-26 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2011-08-30 | PUBLISHED FOR OPPOSITION |
| 2011-08-30 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2011-10-25 | NOA E-MAILED - SOU REQUIRED FROM APPLICANT |
| 2012-04-25 | TEAS EXTENSION RECEIVED |
| 2012-05-16 | CASE ASSIGNED TO INTENT TO USE PARALEGAL |
| 2012-04-25 | EXTENSION 1 FILED |
| 2012-05-16 | EXTENSION 1 GRANTED |
| 2012-05-17 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2012-10-25 | TEAS EXTENSION RECEIVED |
| 2012-10-25 | EXTENSION 2 FILED |
| 2012-10-30 | EXTENSION 2 GRANTED |
| 2012-10-31 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2013-04-25 | TEAS EXTENSION RECEIVED |
| 2013-04-25 | EXTENSION 3 FILED |
| 2013-05-01 | EXTENSION 3 GRANTED |
| 2013-05-02 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2013-10-24 | TEAS STATEMENT OF USE RECEIVED |
| 2013-10-24 | USE AMENDMENT FILED |
| 2013-11-06 | STATEMENT OF USE PROCESSING COMPLETE |
| 2013-11-11 | ALLOWED PRINCIPAL REGISTER - SOU ACCEPTED |
| 2013-11-12 | LAW OFFICE REGISTRATION REVIEW COMPLETED |
| 2013-11-13 | NOTICE OF ACCEPTANCE OF STATEMENT OF USE E-MAILED |
| 2013-12-17 | REGISTERED-PRINCIPAL REGISTER |
| 2018-12-17 | COURTESY REMINDER - SEC. 8 (6-YR) E-MAILED |
| 2020-06-12 | TEAS SECTION 8 & 15 RECEIVED |
| 2020-08-10 | CASE ASSIGNED TO POST REGISTRATION PARALEGAL |
| 2020-08-10 | REGISTERED - SEC. 8 (6-YR) ACCEPTED & SEC. 15 ACK. |
| 2020-08-10 | NOTICE OF ACCEPTANCE OF SEC. 8 & 15 - E-MAILED |