ADIPOGEN - Trademark Details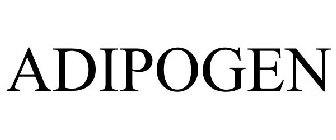
Status: 702 - Section 8 & 15-Accepted And Acknowledged
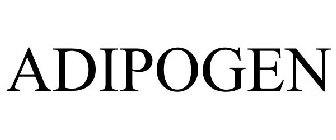
Serial Number
77636854
Registration Number
4146965
Word Mark
ADIPOGEN
Status
702 - Section 8 & 15-Accepted And Acknowledged
Status Date
2018-05-24
Filing Date
2008-12-19
Registration Number
4146965
Registration Date
2012-05-22
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2009-07-14
Attorney Name
Law Office Assigned Location Code
L80
Employee Name
CARL III, FRED
Statements
Indication of Colors claimed
Color is not claimed as a feature of the mark.
Goods and Services
Assays for research purposes; Biochemical proteins, enzymes and antibodies for scientific and medical research; Biochemical reagents commonly known as probes, for detecting and analyzing molecules in protein or nucleotide arrays; Biochemicals, namely, polypeptides for in vitro research use; Biochemicals, namely, polyclonal and monoclonal antibodies for in vitro scientific or research use; Biomedical compounds, namely, peptide substrates used in analyzing and detecting enzymes and proteins for laboratory or research use; Chemicals for use in the purification of proteins for in vitro use; Diagnostic kits for research and in vitro use consisting primarily of polyclonal and monoclonal antibodies, buffers [, and reagents ] to monitor proteins and reagents; Diagnostics, namely, diagnostic preparations and reagents other than for medical or veterinary purposes; Diagnostic preparations and reagents for scientific or research use; Diagnostic preparations and reagents for clinical or medical laboratory use; Diagnostic reagents for in vitro use in biochemistry, clinical chemistry and microbiology; Laboratory chemicals, namely, an antibody reagent used for the detection of antigens in cell and tissue analysis for in vitro diagnostic use; Protein arrays and nucleotide arrays for scientific and medical research; Reagent for chemical analyses; Reagents for scientific or medical research use; Reagents for use in scientific apparatus for chemical or biological analysis; Stem cells and stem cell reagents for research purposes; Testing kits containing peptide substrates used in analyzing and detecting proteins, enzymes and receptors for laboratory or research use; Testing kits containing peptide substrates used in analyzing and detecting proteins, enzymes and receptors for clinical or medical laboratory use
Goods and Services
Biological preparations for medical purposes; Chemical reagents for medical or veterinary purposes; Diagnostic agents, preparations and substances for medical purposes; Diagnostic kits consisting primarily of polyclonal and monoclonal antibodies, buffers, and reagents for use in disease testing; Diagnostic preparations for medical purposes; Diagnostic reagents for medicinal use; Drug testing kits comprised of medical diagnostic reagents and assays for testing body fluids; Medical diagnostic reagents; Pharmaceutical preparations for treating diabetes; Pharmaceutical preparations for the prevention and treatment of disorders of the nervous system, the immune system, the cardio-vascular system, the metabolic system, the respiratory system, the musculo-skeletal system, the genitourinary system, for the treatment of obesity and diabetes and inflammatory disorders, for use in angiogenesis, dermatology, oncology, hematology and in tissue and organ transplantation, in ophthalmology and for gastroenterological disorders [ ; Pharmaceutical preparations and substances for the treatment of infectious diseases, blood disorders, pain, inflammation, sepsis, alopecia, obesity and cognitive disorders; Pharmaceutical preparations and natural medicinal extracts derived from animal, plant and microorganism sources for the treatment of viral, metabolic, endocrine, musculoskeletal, cardiovascular, cardiopulmonary, genitourinary, sexual dysfunction, obesity and diabetes, oncological, hepatological, ophthalmic, respiratory, neurological, gastrointestinal, hormonal, dermatological, psychiatric and immune system related diseases and disorders; Reagents and media for medical and veterinary diagnostic purposes ]
Pseudo Mark
ADIPO GEN
Pseudo Mark
ADIPO GEN
Classification Information
International Class
001 - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry; unprocessed artificial resins, unprocessed plastics; manures; fire extinguishing compositions; tempering and soldering preparations; chemical substances for preserving foodstuffs; tanning substances; adhesives used in industry. - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry; unprocessed artificial resins, unprocessed plastics; manures; fire extinguishing compositions; tempering and soldering preparations; chemical substances for preserving foodstuffs; tanning substances; adhesives used in industry.
US Class Codes
001, 005, 006, 010, 026, 046
Class Status Code
6 - Active
Class Status Date
2008-12-23
Primary Code
001
First Use Anywhere Date
2009-11-01
First Use In Commerce Date
2009-11-01
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
6 - Active
Class Status Date
2009-05-07
Primary Code
005
First Use Anywhere Date
2009-11-01
First Use In Commerce Date
2009-11-01
Current Trademark Owners
Party Name
Party Type
32 - 2nd New Owner Entered After Registration
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Trademark Owner History
Party Name
Party Type
32 - 2nd New Owner Entered After Registration
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
31 - 1st New Owner Entered After Registration
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
10 - Original Applicant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Correspondences
Name
Rob G. Leach
Address
Please log in with your Justia account to see this address.
Trademark Events
| Event Date | Event Description |
| 2008-12-23 | NEW APPLICATION ENTERED IN TRAM |
| 2008-12-24 | NOTICE OF PSEUDO MARK MAILED |
| 2009-03-14 | ASSIGNED TO EXAMINER |
| 2009-03-22 | NON-FINAL ACTION WRITTEN |
| 2009-03-22 | NON-FINAL ACTION E-MAILED |
| 2009-03-22 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2009-05-07 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2009-05-07 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2009-05-07 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2009-06-07 | EXAMINERS AMENDMENT -WRITTEN |
| 2009-06-07 | EXAMINERS AMENDMENT E-MAILED |
| 2009-06-07 | NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED |
| 2009-06-07 | EXAMINER'S AMENDMENT ENTERED |
| 2009-06-07 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2009-06-09 | ASSIGNED TO LIE |
| 2009-06-09 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2009-06-24 | NOTICE OF PUBLICATION |
| 2009-07-14 | PUBLISHED FOR OPPOSITION |
| 2009-10-06 | NOA MAILED - SOU REQUIRED FROM APPLICANT |
| 2009-10-09 | TEAS CHANGE OF CORRESPONDENCE RECEIVED |
| 2009-12-04 | TEAS CHANGE OF OWNER ADDRESS RECEIVED |
| 2009-12-04 | APPLICANT/CORRESPONDENCE CHANGES (NON-RESPONSIVE) ENTERED |
| 2010-04-05 | TEAS EXTENSION RECEIVED |
| 2010-04-05 | EXTENSION 1 FILED |
| 2010-04-05 | EXTENSION 1 GRANTED |
| 2010-04-07 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2010-09-24 | TEAS EXTENSION RECEIVED |
| 2010-10-12 | CASE ASSIGNED TO INTENT TO USE PARALEGAL |
| 2010-09-24 | EXTENSION 2 FILED |
| 2010-10-12 | EXTENSION 2 GRANTED |
| 2010-10-13 | ASSIGNED TO EXAMINER |
| 2010-10-13 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2011-03-28 | TEAS EXTENSION RECEIVED |
| 2011-03-28 | EXTENSION 3 FILED |
| 2011-04-01 | EXTENSION 3 GRANTED |
| 2011-04-02 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2011-10-05 | TEAS EXTENSION RECEIVED |
| 2011-10-05 | EXTENSION 4 FILED |
| 2011-10-19 | EXTENSION 4 GRANTED |
| 2011-10-20 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2012-03-28 | TEAS STATEMENT OF USE RECEIVED |
| 2012-03-28 | USE AMENDMENT FILED |
| 2012-03-30 | STATEMENT OF USE PROCESSING COMPLETE |
| 2012-04-13 | ALLOWED PRINCIPAL REGISTER - SOU ACCEPTED |
| 2012-04-16 | LAW OFFICE REGISTRATION REVIEW COMPLETED |
| 2012-04-17 | NOTICE OF ACCEPTANCE OF STATEMENT OF USE E-MAILED |
| 2012-05-22 | REGISTERED-PRINCIPAL REGISTER |
| 2014-02-12 | AUTOMATIC UPDATE OF ASSIGNMENT OF OWNERSHIP |
| 2017-05-22 | COURTESY REMINDER - SEC. 8 (6-YR) E-MAILED |
| 2017-08-09 | AUTOMATIC UPDATE OF ASSIGNMENT OF OWNERSHIP |
| 2018-05-10 | TEAS SECTION 8 & 15 RECEIVED |
| 2018-05-24 | CASE ASSIGNED TO POST REGISTRATION PARALEGAL |
| 2018-05-24 | REGISTERED - SEC. 8 (6-YR) ACCEPTED & SEC. 15 ACK. |
| 2018-05-24 | NOTICE OF ACCEPTANCE OF SEC. 8 & 15 - E-MAILED |
| 2021-05-22 | COURTESY REMINDER - SEC. 8 (10-YR)/SEC. 9 E-MAILED |
| 2021-08-24 | TEAS SECTION 8 & 9 RECEIVED |
| 2021-09-09 | CASE ASSIGNED TO POST REGISTRATION PARALEGAL |
| 2021-09-09 | OFFICE ACTION ISSUED POU1 |